Given the following equilibrium constants,
Cd(IO3) 2 Ksp = 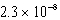
Cd(NH3) 42+ Kf = 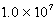
Determine K for the dissolution of the sparingly soluble salt Cd(IO3) 2 in aqueous ammonia (shown below) .Cd(IO3) 2(s) + 4NH3(aq)  Cd(NH3) 42+(aq) + 2IO3-(aq)
Cd(NH3) 42+(aq) + 2IO3-(aq)
Definitions:
Establish Customer Value
The process of determining the benefit that a product or service provides to a customer relative to its cost, enhancing customer attraction and retention.
Maintain Relationships
The ongoing efforts of a business to keep a positive and productive connection with its customers, partners, or employees.
Collaborative Filtering
A method used by recommendation systems to predict the interests of a user by collecting preferences from many users.
Consumer Preferences
The subjective tastes and desires influencing the buying behavior of individuals or groups when choosing among alternative products or services.
Q6: If a chemical reaction is exothermic,but
Q6: For which of the following equilibria does
Q28: The equilibrium constant,K<sub>c</sub>,for the decomposition of
Q30: In an octahedral complex,electrons in <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q33: For a reaction,A <span class="ql-formula"
Q47: At what temperatures will a reaction
Q53: Given that K<sub>a</sub> for the weak
Q65: Strontium oxide has a face centered
Q66: Formulas for derivatives of hydrocarbons may be
Q87: Reduction of a ketone produces a(n)_.<br>A) alkane<br>B)