Given the reaction below, explain how the equilibrium would be reestablished after each of the following stresses are applied.
4NH3(g) 3O2(g) 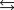 2N2(g) 6H2O(g)
2N2(g) 6H2O(g) 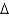 H° 12.0 kJ/mol
H° 12.0 kJ/mol
a) Addition of water
b) Removal of ammonia
c) Increase of the volume of the container
d) Lowering of the reaction temperature
Definitions:
Firm Offer
An offer in a contract that is expressed in a way that it cannot be revoked for a certain period of time.
Merchant
An individual or business entity engaged in the trade of goods, services, or both, to consumers.
Bilateral Contract
A mutual agreement between two parties where each promises to perform an act in exchange for the other party's act.
Valid Contract
A legally binding agreement between parties that is enforceable by law, meeting all essential elements like offer, acceptance, consideration, and mutual intent.
Q43: Calculate the solubility of MgF<sub>2</sub> in water
Q66: The values of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="The values
Q70: Which unit cell contains the most atoms?<br>A)fcc<br>B)bcc<br>C)cubic<br>D)both
Q72: What type of motion is depicted in
Q86: Give an example of a spontaneous and
Q106: Write the expression for the reaction quotient
Q111: Which of these could be the units
Q118: Consider substances that exist as liquids under
Q128: The center of a cubic hole is
Q162: Air (consisting mostly of nitrogen (N<sub>2</sub>), oxygen