Given the reaction below, explain how the equilibrium would be reestablished after each of the following stresses are applied.
4NH3(g) 3O2(g) 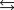 2N2(g) 6H2O(g)
2N2(g) 6H2O(g) 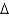 H° 12.0 kJ/mol
H° 12.0 kJ/mol
a) Addition of water
b) Removal of ammonia
c) Increase of the volume of the container
d) Lowering of the reaction temperature
Definitions:
Biological Molecules
Organic compounds essential to life, including carbohydrates, lipids, proteins, and nucleic acids, forming the structure and performing the functions of living organisms.
Fatty Acids
Long-chain hydrocarbons that contain a carboxylic acid group at one end. They are an important component of lipids in living organisms.
Lipids
A broad group of naturally occurring molecules that include fats, waxes, sterols, and fat-soluble vitamins among others, used for energy storage and as structural components of cell membranes.
Organic Acids
Organic acids are a group of acidic compounds that contain carbon and are found in many plants and animals, essential in various biochemical processes.
Q7: Which one of the following A-D is
Q7: Given the following data for the reaction
Q37: Given the following reactions and associated equilibrium
Q42: Solid mercury(II) oxide decomposes when heated to
Q56: For the reaction of hydrogen and nitrogen
Q61: Which of the following refers to an
Q71: A catalyst that is in the same
Q93: Which of the listed perturbations would change
Q126: What is the pH of a solution
Q149: Which of the following requires the smallest