2.0 L of a ideal nitrogen gas (N2)are at 0.00°C and 1.0 atm.The ideal gas constant is 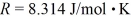 = 0.0821 L • atm/mol • K,Avogadro's number is 6.022 × 1023 molecules/mol,and the ATOMIC mass of nitrogen is 14 g/mol.
= 0.0821 L • atm/mol • K,Avogadro's number is 6.022 × 1023 molecules/mol,and the ATOMIC mass of nitrogen is 14 g/mol.
(a)Determine the number of moles of N2.
(b)How many molecules of N2 are present?
(c)What is the mass of this gas?
Definitions:
Behavioral Style
An individual's typical mode of responding or behaving across different situations; their characteristic manner of acting, reacting, and interacting.
Difficult
Characterized by challenges or obstacles that make a task hard to accomplish.
Chess and Thomas
Refers to Stella Chess and Alexander Thomas, developmental psychologists known for their research on temperament in children.
Goodness of Fit
A concept in psychology that explains how well an individual's temperament matches with their environment, affecting their development and behavior.
Q4: You and your surfing buddy are waiting
Q15: 2.0 L of a ideal nitrogen gas
Q21: During a collision with a wall,the velocity
Q24: What is the mass density of argon
Q27: A billiard ball traveling at 3.00 m/s
Q27: In the figure,a uniform rectangular crate 0.40
Q32: A uniform magnetic field of magnitude 0.80
Q34: A multiloop circuit is shown in the
Q43: The entropy of an isolated system must
Q44: A sealed container holds 0.020 moles of