The system is in equilibrium with the concentrations as shown at 222 C:
2 NH₃(g) 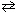 N₂(g) + 3 H₂ (g) Equilibrium pressure 0.4 atm 0.2 atm 0.6 atm By means of a cold trap, ammonia is removed until its equilibrium partial pressure is 0.1 atm. As this change occurs:
N₂(g) + 3 H₂ (g) Equilibrium pressure 0.4 atm 0.2 atm 0.6 atm By means of a cold trap, ammonia is removed until its equilibrium partial pressure is 0.1 atm. As this change occurs:
Definitions:
Tuberculin Test
A diagnostic test for tuberculosis using an intradermal injection to measure the body's immune response to the bacterium.
Needle Angle
The angle at which a needle is inserted into the skin or vein for injections or blood withdrawal, critical for ensuring proper needle placement.
27-Gauge
Describes the diameter of needles, with 27-gauge indicating a very fine needle size commonly used in medical procedures.
Phenytoin
An anticonvulsant medication used to control seizures, particularly in epilepsy.
Q17: Calculate the pH of a solution after
Q31: Which of the following reactions is product
Q32: The following reduction of Fe<sub>2</sub> O<sub>3</sub> to
Q33: The diagram below is a time-concentration curve
Q82: Exhibit 11-2 The phase diagram below is
Q90: For the Haber reaction at equilibrium ,
Q135: After completing the following unfinished Base Ionization
Q140: Hypochlorous acid (HClO) has an acid ionization
Q164: Some N₂H<sub>4</sub> is placed in a 1.0
Q183: The solubility of ethylene (C<sub>2</sub>H<sub>4</sub>) in water